At a recent Faculty Lunch Seminar Series, Professor Joel Bernstein talked about his experience as an expert witness over the past two decades. The following exchange grew out of that presentation.
Did you ever think that becoming an expert witness was part of your career path?
When I finished my PhD at age 26, I wanted to pursue a career in academia doing basic science. I knew nothing about expert witnessing; patents and patent law seemed totally remote and boring. One day in the autumn of 1991, I received a phone call. The lawyers on the other end of the line had a lot of questions. That is how it all started, and I became an expert witness for Glaxo in a patent infringement case involving Zantac, at the time the world’s largest selling drug at US$ 3.5 billion/year (~US$ 10 million/day). I have been doing it ever since.
Is being an expert witness today still as thrilling as it was over twenty years ago?
Very much so. While there are many similarities, every case is unique. In a comment to The Economist, one patent lawyer was quoted as saying “Forget horse racing; patent litigation is the true sport of kings.” I agree. This is the ultimate intellectual challenge that takes place at the intersection of two very different modes of thinking. In each case, the lawyers and I educate each other to develop a legal strategy in which as an expert witness, I can present and defend the science to make the best case possible. Learning to think like and with lawyers is a challenging and rewarding task for someone like me. After all, as an academic I am basically a teacher, and they are extremely motivated students.
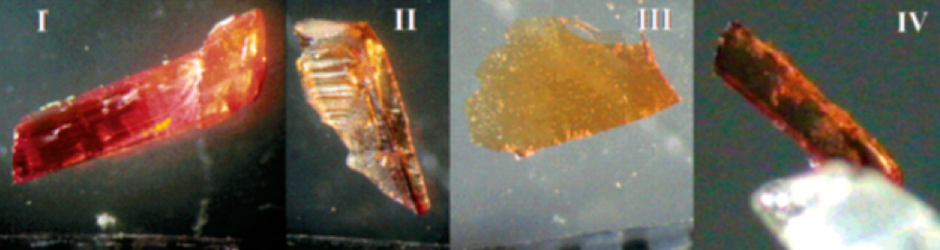
Doesn’t it come always down to specific characteristics and precise amounts?
Chemistry is not as exact a science as most people – and even many chemists - tend to think. Most patent cases boil down to a ”battle of the experts”. As in a criminal trial, expert witnesses in patent litigation are called upon by both sides to help determine whether “spectra” or “X-ray powder diffraction patterns” (i.e. the “fingerprints” of materials) match those of patented material. Experts – often scientific colleagues, and at times even collaborators – resort to what we call (non-pejoratively) “fuzzy logic” and the recognition of patterns. Some people may claim that experts can be “bought” by their clients, but my own feeling is that most often these differences in opinion represent genuine differences in interpretation, based on experience and the scientist’s view of the issue, and the role of fuzzy logic in chemistry.
So, what you see depends on how you look at it?
In some senses yes, but definitely not always. In fact, fuzzy logic is one of the manifestations of the famous wave-particle duality in physics. Chemists are trained very early in their education to use fuzzy logic. An example from my freshman course will help illustrate this point. In chemistry we can readily define what we mean by a covalent bond (as say in the hydrogen molecule) as one that is formed by the sharing of two electrons between two atoms. On the other hand there is something called the ionic bond (as in table salt – sodium chloride) that is based on the attraction between positively and negatively charged particles called ions. However, it turns out that very few bonds are purely covalent or purely ionic. So how do we describe it? Using the ideal models and fuzzy logic we say that a particular bond is, say, covalent with a certain “percentage of ionic character”. The percentage of ionic character is very much a qualitative, or fuzzy concept -first proposed nearly a century ago by the Nobel Prize winning pioneering chemist Linus Pauling - that chemists find extremely useful to, say, compare and predict the properties of substances.
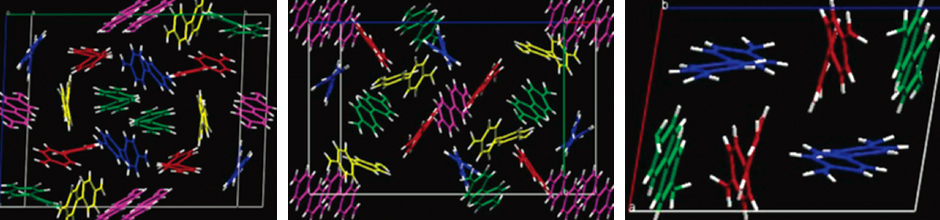
A chemist is also a detective at times. Is that true?
“Detect” is a word that pervades the discipline of chemistry, but not always in the forensic sense. How can we detect and measure the impurities in our air and our water? Then how can we develop methods to minimize the deleterious material and purify the desired one? How pure must our drugs be? What impurities are present? Do they lead to undesirable side effects? The chemical detective work pervades almost every aspect of life: How can we non-intrusively detect explosive materials carried by potential terrorists? How much performance-enhancing drug is in the blood of a competitive athlete? The questions are endlessly challenging, and getting the answers to them comprise the satisfying and rewarding aspects of a career in chemistry.
What have you learned from working with lawyers that you did not already know?
A great deal. First of all there is simply the difference in the way scientists and lawyers approach a patent case, which of course has a scientific basis – otherwise I wouldn’t be involved. Then there is the way of presenting it and defending it, the nuances of language and how they differ between scientists and the courts. While in principle patents and scientific publications are both intended to disseminate knowledge, in the end they serve different purposes and hence their construction, language and content are very different. Recognizing and understanding these differences are part of the intellectual and professional interplay between experts and lawyers.

How has chemistry changed during your distinguished career?
Since I started graduate school in 1962, chemistry – or at least the chemistry that I do - has developed very much in parallel with the revolution in electronics and computers. All of our instrumentation has gone from analog to digital. The incredible amounts of information that we are generating are readily available in databases that are accessible and analyzable. Regular trips to the library to catch up on the literature have been replaced by accessing libraries from virtually anywhere in the world on a laptop. The impact of computerization in my specific area of chemistry – X-ray crystallography – is staggering. Graduate student colleagues of mine earned a PhD by carrying out one or two crystal structure determinations in 4-5 years. Today, the same task can be accomplished in less than one hour. This kind of progress--to say nothing about our incredible ability to design and prepare new materials, including new drugs, with fantastic, indeed previously unfathomable properties--has pervaded all of chemistry.
Back in the lab, what questions are you pondering today?
In spite of tremendous progress in my field of crystallography, there are some fundamental questions that still need answers. For instance, while we manage to grow marvelous crystals for many purposes, we still don’t understand how - starting from a random solution of molecules - a crystal growth process begins and proceeds to become, say, the sucrose crystals that fill our sugar bowl. We can neither predict nor prescribe exactly how to form crystals, what the structure of a crystal might be (if we succeed in obtaining it), and what their properties will be if crystals do actually form. I don’t find this situation frustrating at all. In the course of my career we have made significant progress in our understanding and in our control of many systems but there are plenty of fascinating challenges for quite a few future generations of chemists.